This event features two days of interactive workshops, presentations, panels and roundtables, all developed for smaller companies with limited resources.
Join us to gain a comprehensive education on clinical risk management and the tools to assess, maintain and mitigate risk to ensure compliance.
Lorem ipsum dolor sit amet consectetur adipiscing elit. Quisque faucibus ex sapien vitae pellentesque sem placerat. In id cursus mi pretium tellus duis convallis. Tempus leo eu aenean sed diam urna tempor. Pulvinar vivamus fringilla lacus nec metus bibendum egestas. Iaculis massa nisl malesuada lacinia integer nunc posuere. Ut hendrerit semper vel class aptent taciti sociosqu. Ad litora torquent per conubia nostra inceptos himenaeos.
Lorem ipsum dolor sit amet consectetur adipiscing elit. Quisque faucibus ex sapien vitae pellentesque sem placerat. In id cursus mi pretium tellus duis convallis. Tempus leo eu aenean sed diam urna tempor. Pulvinar vivamus fringilla lacus nec metus bibendum egestas. Iaculis massa nisl malesuada lacinia integer nunc posuere. Ut hendrerit semper vel class aptent taciti sociosqu. Ad litora torquent per conubia nostra inceptos himenaeos.
Lorem ipsum dolor sit amet consectetur adipiscing elit. Quisque faucibus ex sapien vitae pellentesque sem placerat. In id cursus mi pretium tellus duis convallis. Tempus leo eu aenean sed diam urna tempor. Pulvinar vivamus fringilla lacus nec metus bibendum egestas. Iaculis massa nisl malesuada lacinia integer nunc posuere. Ut hendrerit semper vel class aptent taciti sociosqu. Ad litora torquent per conubia nostra inceptos himenaeos.
Lorem ipsum dolor sit amet consectetur adipiscing elit. Quisque faucibus ex sapien vitae pellentesque sem placerat. In id cursus mi pretium tellus duis convallis. Tempus leo eu aenean sed diam urna tempor. Pulvinar vivamus fringilla lacus nec metus bibendum egestas. Iaculis massa nisl malesuada lacinia integer nunc posuere. Ut hendrerit semper vel class aptent taciti sociosqu. Ad litora torquent per conubia nostra inceptos himenaeos.
Lorem ipsum dolor sit amet consectetur adipiscing elit. Quisque faucibus ex sapien vitae pellentesque sem placerat. In id cursus mi pretium tellus duis convallis. Tempus leo eu aenean sed diam urna tempor. Pulvinar vivamus fringilla lacus nec metus bibendum egestas. Iaculis massa nisl malesuada lacinia integer nunc posuere. Ut hendrerit semper vel class aptent taciti sociosqu. Ad litora torquent per conubia nostra inceptos himenaeos.
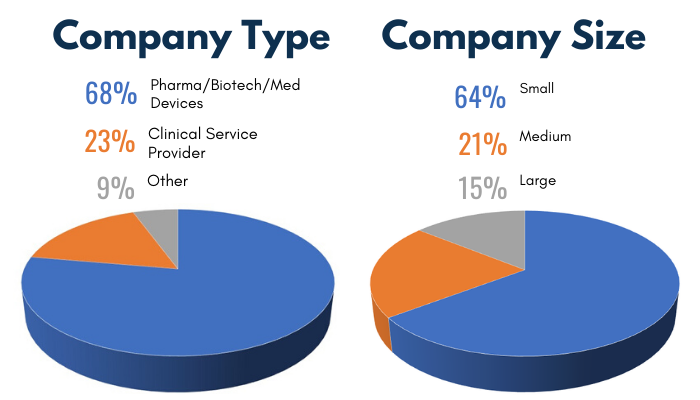
2019 Audience Demographics
TOP REASONS TO ATTEND
Having successfully established a series of clinical quality events on the East Coast and in Europe, ExL Events brought their well-known conference series to San Francisco in 2019, with a Clinical Trial Risk Management Seminar geared towards biotechnology and biopharmaceutical companies. With a successful first year under our belts, we are excited to come back and continue to provide the educational needs for smaller companies with limited resources.
- Understand the value of risk-based approaches to ensure compliance using limited resources.
- Ensure your clinical quality risk management plan covers all the critical elements to ensure GCP compliance.
- Develop an effective strategy to work with and oversee your CRO partners to ensure transparency, compliance and productivity.
- Learn the tools to achieve a constant state of inspection-readiness.
Who Should Attend
This conference is designed for professionals from pharmaceutical, biotechnology and medical device companies; CROs; and other clinical trial service providers whose responsibilities involve the following:
- Good Clinical Practice (GCP)
- Clinical Quality Assurance (CQA)
- Clinical Quality Control (CQC)
- Clinical Trial Operations/Management
- Clinical Research
- Quality Management/ Global Quality Management
- Audits/Inspections
- Compliance/Global Compliance
- Data Management/Systems Operations
- Clinical Monitoring
- Regulatory Affairs
- Safety and Risk Management/Operations
The event is also relevant to clinical QA, compliance and operations professionals from:
- Quality Service Providers and Consulting Companies
- CROs
- Central, Imaging and ECG Labs
- Investigative Sites
- IRBs
- Data Management and Software Vendors
- Safety Reporting Vendors